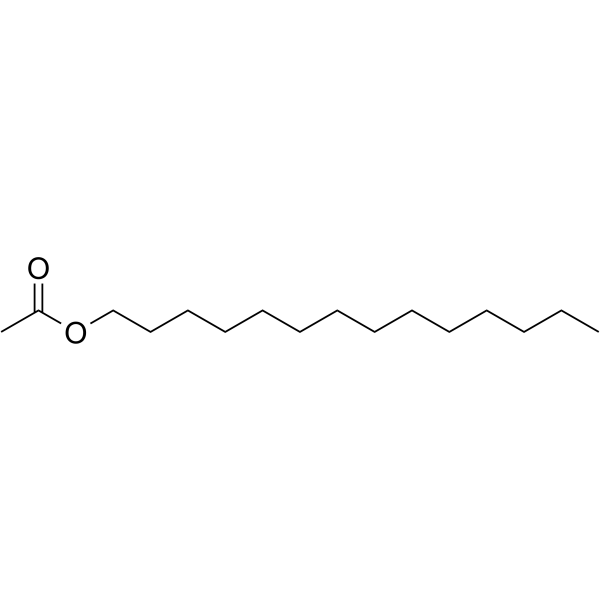
Tetradecyl acetate
CAS No. 638-59-5
Tetradecyl acetate( —— )
Catalog No. M28332 CAS No. 638-59-5
Tetradecyl acetate is a sex pheromone produced by Ctenopseustis obliquana females that disrupts the mating of pest species.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 500MG | 37 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameTetradecyl acetate
-
NoteResearch use only, not for human use.
-
Brief DescriptionTetradecyl acetate is a sex pheromone produced by Ctenopseustis obliquana females that disrupts the mating of pest species.
-
DescriptionTetradecyl acetate is a sex pheromone produced by Ctenopseustis obliquana females that disrupts the mating of pest species.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptormitoNEET
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number638-59-5
-
Formula Weight256.43
-
Molecular FormulaC16H32O2
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (389.99 mM)
-
SMILESCCCCCCCCCCCCCCOC(C)=O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Shi G, et al. TT01001 attenuates oxidative stress and neuronal apoptosis by preventing mitoNEET-mediated mitochondrial dysfunction after subarachnoid hemorrhage in rats. Neuroreport. 2020 Aug 5;31(11):845-850.
molnova catalog



related products
-
[pY185]MAP (177 - 18...
[pY185]MAP (177 - 189) kinase
-
7,12-DIMETHYLBENZ[A]...
7,12-Dimethylbenz[a]anthracene is a highly potent carcinogen that is activated by microsomal enzymes to a diol epoxide metabolite that binds covalently to DNA in mammalian cells, leading ultimately to tumor induction.
-
A2AR-agonist-1
A2AR-agonist-1 is a potent A2AR and ENT1 agonist (Ki: 4.39 and 3.47 for A2AR and ENT1. It targets the Adenosine A2A Receptor and Adenosine Transporter for Neuroprotection.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


